Biography
Sanjiv Luther studied cell biology at the ETH in Zürich. He received his PhD in 1996 from the University of Lausanne for his work on anti-viral immune responses in the laboratory of Hans Acha-Orbea. He then moved to the laboratory of Jason Cyster at the Howard Hughes Medical Institute at the University of California, San Francisco where he investigated the role of chemotactic factors in lymphoid tissue development and function. In 2003 he joined the Department of Immunobiology (formerly Biochemistry) as Assistant Professor funded by a career-development award from the Swiss National Science Foundation. In 2009 he became Associate Professor and in 2021 Full Professor. His current research focuses on the characterization of fibroblastic stromal cells found within secondary lymphoid organs and sites of chronic inflammation or cancer.
Research Interests
Lymphoid tissue fibroblasts in health and disease
Secondary lymphoid organs, such as lymph nodes and spleen, are critical sites for mounting and regulating lymphocyte responses and thereby act like the ‘brain’ for adaptive immunity. On one hand these organs drain signals and cells derived from sites of infection or tumors, on the other hand they allow the recirculation of lymphocytes and the selection of the rare antigen-specific B and T lymphocytes eventually leading to their local proliferation and differentiation into effector cells. Similar to the brain, these organs are highly compartmentalized to allow different processes to occur efficiently. The compartmentalization into a B and T zone is established by distinct fibroblastic stromal cell subsets that form these microenvironments, most notably follicular dendritic cells (FDC) within the B cell follicles and fibroblastic reticular cells within the T cell zone. While we have made much progress in understanding the biology of the mobile DC and T lymphocytes, we still know relatively little about the resident fibroblasts that form these ‘niches’. Given the critical role of lymph nodes in adaptive immunity our goal is to improve our understanding of these fibroblasts by investigating their development, heterogeneity and function in health and disease, using modern genetic tools. We believe that this will help us to to improve adaptive immune responses to vaccines, infections and cancers, or to dampen them in situations of autoimmunity.
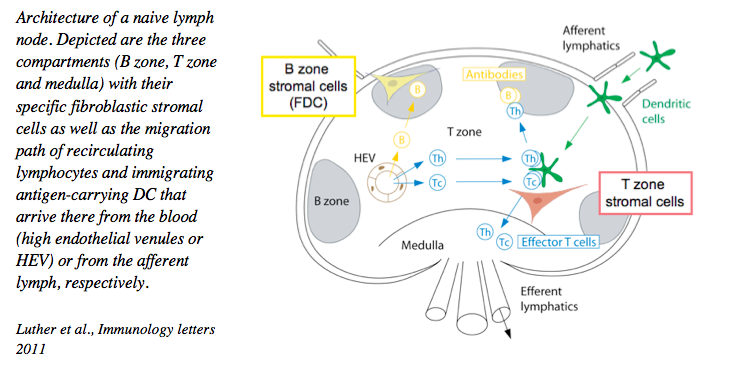
1. Fibroblast heterogeneity and their role in creating niches for immune cells and immune-related processes
Over the past years we have developed the technology to isolate, culture, manipulate and characterize lymphoid tissue fibroblasts at the phenotypic and functional level, in vitro and in vivo. We could demonstrate that these cells are not a simple backbone of these organs, but FRC are important players regulating the biology of dendritic cells, T cells and B cells, such as their migration, survival and response. These cells form a relatively rigid 3-dimensional cell network used for lymphocyte migration. Despite this property they are highly reactive and plastic, allowing a rapid change in their activity within hours of vaccination, and an increase in number during lymph node swelling typical for the first few days of an immune response.
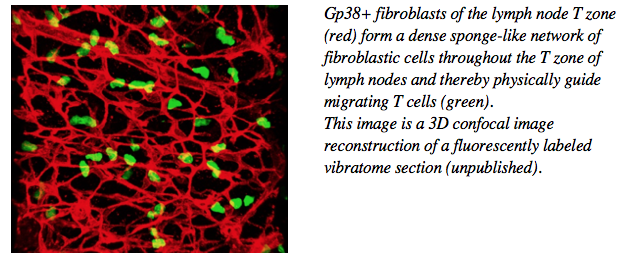
We have characterized the stromal cell niches within the lymph node B zone, T zone and medulla, as well as tertiary lymphoid structures. T zone FRC for example form niches for both T cells as well as dendritic cells thereby bringing these cell types into physical contact and allowing MHC-restricted T cell activation. In the medulla, another FRC subset forms niches for plasma cells thereby promoting their correct localization and survival. More recently, advanced flow cytometry and single cell RNA sequencing uncovered the existence of more than 8 functionally diverse fibroblast subsets in lymph nodes alone, supposedly generating many more niches for specific immune cell processes than only B and T zones and medulla.
Tertiary lymphoid structures arising in various disease settings such as chronic inflammation and autoimmunity harbor also B and T zone fibroblast networks to retain and organize immune cells as well as presumably to regulate their function.
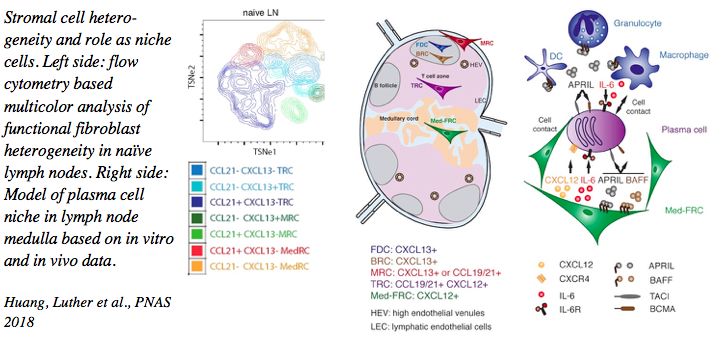
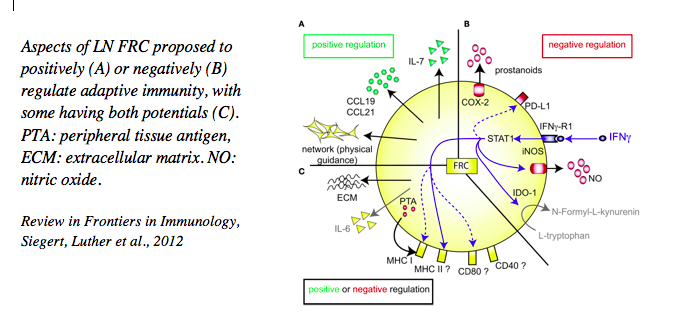
2. Fibroblast-derived factors that regulate adaptive immunity and can be targeted therapeutically
The various FRC subsets are characterized by the expression of many factors relevant for immune cells which have been the focus of much of our work. Besides their constitutive expression of extracellular matrix proteins necessary for organ stability and of chemotactic factors (such as CCL19, CCL21 and CXCL13) allowing organ compartmentalization, we have shown that they express many other cytokines (such as IL-7, IL-6, BAFF, April, CXCL12, IL-33), surface ligands (DLL1, DLL4) and soluble mediators (nitric oxide, prostaglandin E2) that regulate immune cell development, homeostasis and activity, with this regulation being both positive and negative. We could also demonstrate that these cells express several receptors to sense signals derived from neighboring cells, such as LTab, TNFa, IFNg, IL-4, IL-13 and IL-22 to name a few. These studies have revealed an intimate crosstalk between these stromal cells with their hematopoietic as well as endothelial neighbors that is central for ensuring efficient adaptive immunity. In collaborative studies, we have targeted some of these signals in disease settings, such as in lymphocytic tissue infiltration (LTab, TNFa), virus-induced autoimmunity (IL-22), chronic infection with viruses or worms (Cox2/PGE2, LTab) or graft versus host disease (DLL1/4) demonstrating their effectiveness in modulating adaptive immunity. In other collaborative studies we have demonstrated the destruction or alteration of this stromal cell network, such as upon infection with LCM-virus or worms, leading to immunosuppression, again pointing to the critical role of FRC and secondary lymphoid organs as a whole in effective immune defense.
Perspectives
Our present and future studies aim at improving our understanding of the various FRC subsets in different lymphoid organs (lymph nodes, spleen) and effector sites (intestinal lamina propria, cancer tissue) in the context of diverse immune response settings (infection, cancer, graft versus host disease). To that end, we are using cutting edge tools, both in vitro and in vivo, including cre-mediated gene targeting, sophisticated disease models, multicolor flow cytometry, various imaging techniques, gene arrays, RNA sequencing and 3-dimensional cell culture etc.. Various collaborators (listed below) and institutional technology platforms reinforce our team and bring in additional important expertise and tools, in addition to allowing us the validation our findings on human tissues. While one goal of our ongoing projects is to understand the basic biology of FRC in health and in preclinical disease models, our other goal is to design novel intervention strategies targeting the stromal cells rather than immune cells, namely by interfering with fibroblast-based niches critical for immune cell processes, either in lymphoid organs, tertiary lymphoid structures or diseased tissues.
Current collaborators
Petr Broz (Univ. of Lausanne), Chris Buckley (Univ. of Oxford, UK), Tom Cupedo (Rotterdam), Nicolas Fasel (Univ. of Lausanne), Werner Held (Univ. of Lausanne), Melita Irving and George Coukos (Univ. of Lausanne), Burkhard Ludewig (Kantonsspital St.Gallen), Doron Merkler (Univ. of Lausanne), Tanya Petrova (Univ. of Lausanne), Daniel Pinschewer (Univ. of Basel), Freddy Radtke (EPFL), Pascal Schneider (Univ. of Lausanne), Michael Sixt (MPI Munich), Fabienne Tacchini-Cottier (Univ. of Lausanne), Margot Thome (Univ. of Lausanne), Shannon Turley (Genentech, USA) and others.
Job openings
Spontaneous applications are welcome from prospective PhD students and Post-doctoral scientists. Although funding may be available to support positions, all applicants are strongly encouraged to seek external sources of funding.
Please send an email describing why you are interested in joining our group and a CV (including publications) to Sanjiv.Luther@unil.ch
| Person | Position | Contact |
|---|---|---|
| Nagham Alouche | Postdoctoral Fellow | Unisciences |
| Naïma Böllenrücher | PhD Student | Unisciences |
| Chloé Chapuis | Apprentice | chloe.chapuis.2@unil.ch |
| Stéphanie Favre | Technician | Unisciences |
| Marija Jokic | PhD Student | Unisciences |
| Leonor Morgado | Laboratory Support | Unisciences |
| Leonardo Scarpellino | Technician | Unisciences |
| José Villegas Vazquez | Postdoctoral Fellow | Unisciences |


