Biography
Alexis Jourdain received his PhD in 2013 from the University of Geneva for his work on mitochondrial gene expression and mitochondrial RNA granules under the supervision of Prof. Jean-Claude Martinou, as a fellow of the Roche Research Foundation. In 2015, he joined the laboratory of Prof. Vamsi Mootha, Howard Hughes Medical Institute (HHMI) investigator, at the Broad Institute of MIT & Harvard, Harvard Medical School and the Massachusetts General Hospital. During his postdoctoral training as a fellow of the European Molecular Biology Organization (EMBO) and the Swiss National Science Foundation (SNSF), Alexis used systems biology approaches to discover nuclear genes involved in energy metabolism, including genes encoding mitochondrial and pre-mRNA splicing subunits. In spring 2021, Alexis joined the Department of Immunobiology (formerly Biochemistry), at the University of Lausanne as a tenure-track Assistant Professor.
Research Interests
The Jourdain laboratory aims at understanding how cells in our body tune the expression of their genes in response to changes in nutrient levels and metabolic needs, and how this may be dysregulated in disease conditions.
Specifically, we study mitochondria and energy metabolism in the context of cellular differentiation, immunity and metabolic disorders.
Candidates for a postdoctoral fellowship (e.g. EMBO, HFSP) are encouraged to contact us directly.
GENETIC CONTROL OF ENERGY METABOLISM AND MITOCHONDRIAL PLASTICITY
Mitochondrial oxidative phosphorylation (OXPHOS) and glycolysis are the two major pathways for ATP production – or energy metabolism - in human. The reliance on these pathways and the abundance and composition of mitochondria are known to differ across tissues, and may vary during processes such as cellular differentiation and immune response. Shifts in the OXPHOS/glycolysis balance are also seen in pathologies such as cancer and metabolic syndromes (mitochondrial disorders). At present, the full set of molecular mechanisms that support and control mitochondrial biogenesis and energy metabolism is not known. Understanding how these programs function holds promise for diagnosis and therapies by pointing to vulnerabilities that could either be corrected to promote healthy metabolism and immune function, or exploited to starve tumors.
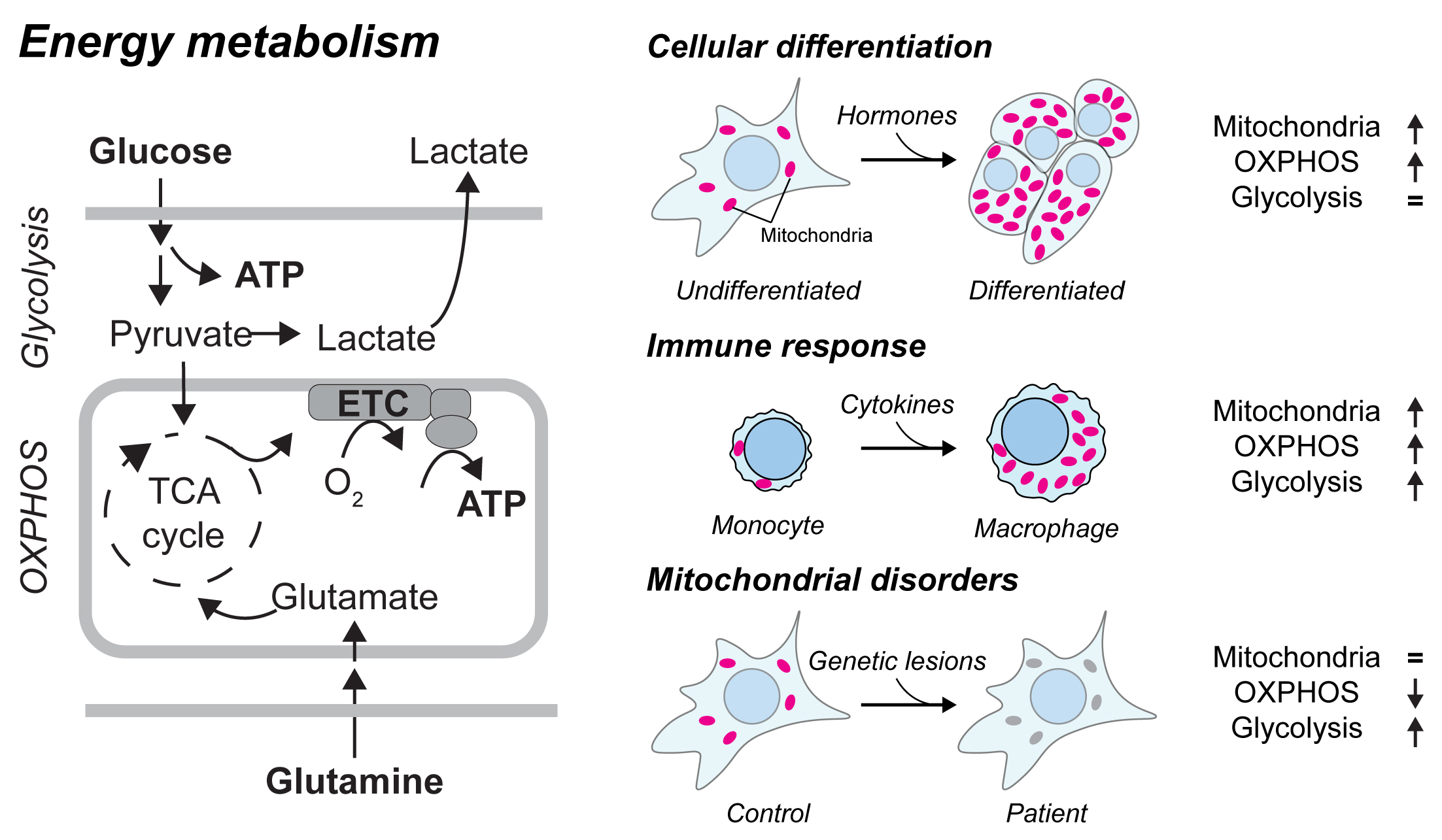
Figure 1: Energy metabolism pathways and metabolic shifts in physiology and pathology. Left: schematic representation of glycolysis and mitochondrial oxidative phosphorylation (OXPHOS), the two main ATP-generating pathways in human cells. Right: three examples of shifts in metabolic programs and/or in mitochondrial abundance. Adapted from Jourdain et al., Molecular Cell 2021.
Our group uses large-scale systems approaches such as genome-wide CRISPR/Cas9 screening, metabolomics and quantitative proteomics (Arroyo*, Jourdain* et al., Cell Metabolism 2016 ; Jourdain et al., Molecular Cell 2021), as well as biochemistry and cell biology techniques, to discover novel mechanisms involved in mitochondrial biogenesis and energy metabolism. Our long-term goals are to:
- Inventory the genes and regulatory networks necessary to support energy metabolism. Knowing the identity of these genes will not only advance our understanding of fundamental cellular metabolism but also help prioritize genes for the diagnosis of suspected mitochondrial disorders, a large and heterogenous group of debilitating conditions for which no therapy currently exists.
- Discover novel mechanisms that support dynamic remodeling in mitochondria and energy metabolism. We use models of cellular differentiation, immune activation and nutrient modulation to identify and manipulate gene expression pathways that control the abundance and composition of mitochondria as well as the expression of metabolic enzymes and transporters
- Study metabolic shifts in pathologies. We aim to apply our findings to understand, and potentially treat, pathologies characterized by metabolic shifts, including mitochondrial disorders, cancers and immune-related disorders.
METABOLIC REGULATION OF MITOCHONDRIAL GENE EXPRESSION
Mitochondria contain a relic of their bacterial past: a small, circular genome called the mitochondrial DNA (mtDNA). In humans, this small DNA molecule encodes only 37 genes that are expressed in the organelle, including 13 genes encoding core subunits of the respiratory chain that are essential for OXPHOS. How expression of mtDNA-encoded genes is regulated and how it responds to environmental stimuli is incompletely understood. This important question demands investigation since a large fraction of the genetically inherited mutations that cause mitochondrial disorders are mtDNA-related. Furthermore, it has recently become clear that mtDNA and its transcripts are frequently released from mitochondria into the cytosol where they trigger pathways of innate immunity and contribute to inflammation and the antiviral response through mechanisms that remain elusive.
Recently, we discovered the existence of “mitochondrial RNA granules” (MRGs) (Jourdain et al., Cell Metabolism 2013 ; Jourdain et al., Cell Reports 2015). We and others provided proof-of-concept evidence that these structures play a crucial role in mtDNA expression by serving as RNA post-transcriptional factories for newly-made mitochondrial transcripts. Exciting questions remain to be answered on the composition and function of these intra-mitochondrial structures.
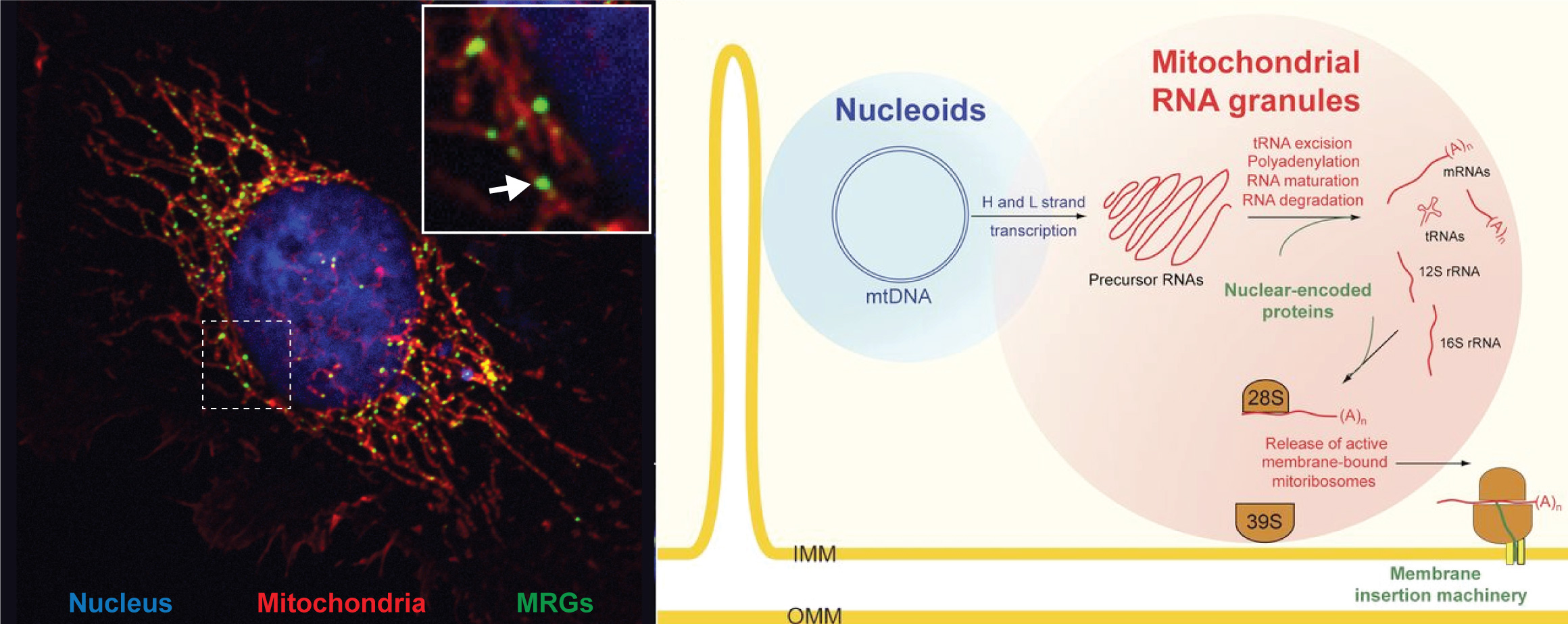
Figure 2: Mitochondrial gene expression and mitochondrial RNA granules (MRGs). Left: a human cell in which the nucleus, the mitochondrial network and the MRGs (arrow) were labelled with fluorescent dyes. Right: model of mitochondrial gene expression in MRGs. Adapted from Jourdain et al., Journal of Cell Biology 2016.
Our laboratory uses genomics, biochemistry and cell biology approaches to characterize the mechanisms of mitochondrial gene expression and their dysfunction in mitochondrial disorders, with a particular emphasis on mitochondrial RNA granules. Our long-term goals are to:
- Discover novel genes involved in mitochondrial gene expression, using large-scale genomics and biochemical technologies, and study their roles in mitochondrial pathologies.
- Characterize the genetic-biophysical properties of mitochondrial RNA granules, by quantitatively studying these structures using simultaneous super-resolution microscopy and genome perturbation.
- Understand how mitochondrial gene expression is impacted by the metabolic state of the cell, including in innate immunity, by combining state of the art gene expression analysis, nutrient source variation and metabolic studies in immune cells (immunometabolism).
Representative publications
Research articles
Jourdain AA#, Begg BE, Mick E, Shah H, Calvo SE, Skinner OS, Sharma R, Blue SM, Yeo GW, Burge CB, Mootha VK#. Loss of LUC7L2 and U1 snRNP Subunits Shifts Energy Metabolism from Glycolysis to OXPHOS. Molecular Cell 2021 (#corresponding authors)
Previewed in
Hoskins & Rhoads, When cells are down on their LUC7L2, alternative splicing rewires metabolism for OXPHOS. Molecular Cell 2021
Arroyo JD*, Jourdain AA*, Calvo SE, Ballarano CA, Doench JG, Root DE, Mootha VK. A Genome-wide CRISPR Death Screen Identifies Genes Essential for Oxidative Phosphorylation. Cell Metabolism 2016 (*first authors)
Jourdain AA, Koppen M, Rodley CD, Maundrell K, Gueguen N, Reynier P, Guaras AM, Enriquez JA, Anderson P, Simarro M and Martinou JC. A mitochondria-specific isoform of FASTK is present in mitochondrial RNA granules and regulates gene expression and function. Cell Reports 2015
Jourdain AA, Koppen M, Wydro M., Rodley CD, Lightowlers RN, Chrzanowska-Lightowlers ZM, Martinou JC. GRSF1 regulates RNA processing in mitochondrial RNA granules. Cell Metabolism 2013
Reviews
Jourdain AA, Popow J, de la Fuente MA, Martinou JC, Anderson P, Simarro M. The FASTK family of proteins: emerging regulators of mitochondrial RNA biology. Nucleic Acids Research 2017
Jourdain AA, Boehm E, Maundrell K, Martinou JC. Mitochondrial RNA granules: Compartmentalizing mitochondrial gene expression. Journal of Cell Biology 2016
Our complete publication list can be found on PubMed and Google Scholar.

