Biography
Petr Broz received his PhD in Microbiology in 2006 from the University of Basel working on the structure of bacterial type 3 secretion systems with Guy R. Cornelis. After working in immunology research for CSL Behring, he joined the laboratory of Denise Monack at Stanford University in 2008, where he started to work inflammasome complexes in the context of bacterial infections. He returned to Basel as an SNSF Assistant Professor in 2013, investigating links between cell-autonomous immunity and inflammasome activation. He joined the Department of Immunobiology (formerly Biochemistry) as an Associate Professor in October 2017 and he became Full Professor in February 2022. His current research focuses on host defense mechanisms, inflammasomes and the induction of pyroptosis, a lytic, inflammatory cell death.
Research Interests
INNATE IMMUNITY AGAINST INTRACELLULAR PATHOGENS
The innate immune system provides the first line of defense against infections by rapidly recognizing and eliminating invading microbes. An important component of innate immunity are pattern recognition receptors, which are sensors that constantly monitor the extracellular and intracellular space of host cells for molecules of bacterial origin. Once the presence of bacteria is detected, these sensors initiate an inflammatory response.
Assembly and regulation of inflammasomes
A subset of these pattern recognition receptors initiates the assembly of cytosolic multi-protein complexes called inflammasomes. The inflammasome complex is a large filamentous assembly formed by progressive oligomerization of receptors and adaptor proteins and serves as an activation platform for inflammatory caspases, a group of cysteine proteases. The prototypic caspase activated by canonical inflammasomes in caspase-1, which control the release of cytokines like IL-1b. Uncontrolled activation of the inflammasome by gain-of-function mutations or sterile inflammatory triggers is an underlying cause of many (auto-) inflammatory diseases. By characterizing new players and regulatory mechanism that control complex assembly and downstream signaling, we aim at identifying new targets for therapeutic modulation of inflammasome activity.
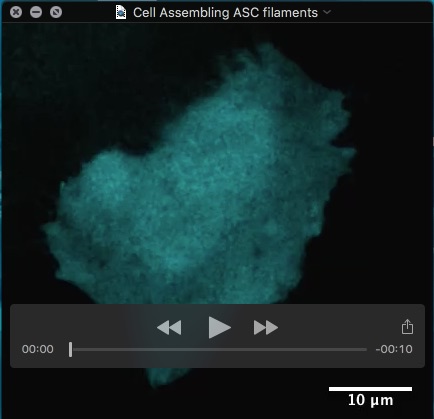
Movie: HeLa cell assembling filaments of the inflammasome adapter protein ASC after receptor engagement.
Induction of pyroptotic cell death by gasdermins
Inflammatory caspases also induce a lytic type of cell death called pyroptosis that restricts pathogen replication. Pyroptosis was recently shown to be caused by gasdermin-D, a member of the newly identified gasdermin protein family. Caspase-dependent cleavage of gasdermin-D creates a cytotoxic N-terminal fragment that targets the plasma membrane where it forms large oligomeric pores. Gasdermin-D pores disrupt the electrochemical gradient and the ability of cells to maintain osmotic balance and stabilize cell volume, leading to swelling, membrane ballooning and ultimately to the lysis of the cell. We study how gasdermins are activated and how they kill cells during bacterial infections by combining cell biology, biochemistry and real-time imaging.
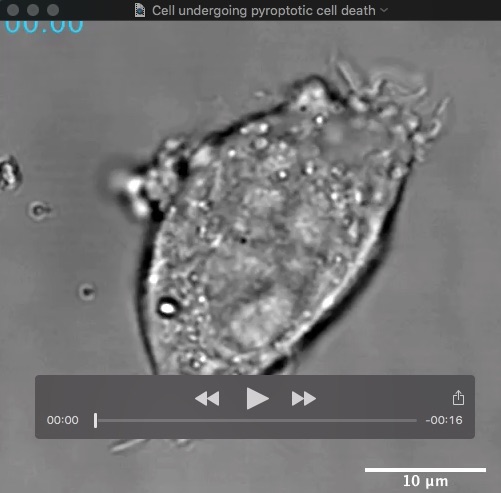
Movie: HeLa cell undergoing pyroptotic cell death following caspase-11-driven activation of gasdermin-D
Interferon-induced GTPases in cell-autonomous immunity
Since inflammasomes are important mediators of inflammation, assembly of these complexes is very tightly regulated and often requires previous induction of other signaling pathways. Activation of inflammasomes in response to Gram-negative bacterial infections requires the production of type-I-interferons, a class of cytokines that regulates the induction of several thousands of genes involved in various aspects of host defense. In particular interferons induce a number of genes involved in cell-autonomous immunity, i.e. processes that allow cells to fight and eliminate pathogens on a single cell level. Among the most highly induced are several families of interferon-induced GTPases, which have been shown to be required to control intracellular killing bacterial and protozoan pathogens. We study how these GTPases recognize intracellular bacteria and how this promotes the activation of inflammasome complexes.
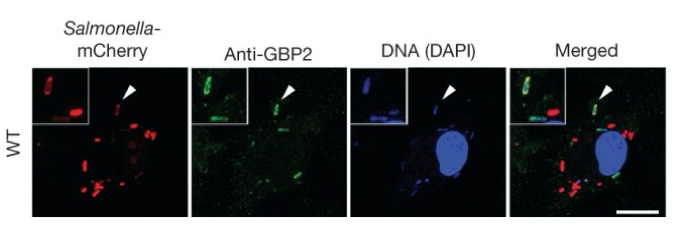
Interferon-induced GTPases (green) attack intracellular Salmonella (mCherry, red). Inset shows Salmonella that have been killed and begin to loose mCherry expression. Scale bars 10 um.
Postdocs
Etienne Meunier, Postdoctoral Fellow, 2013-2016; currently Group Leader, Institut de Pharmacologie et de Biologie Structurale (IPBS), Toulouse, France
Dave Boucher, Postdoctoral Fellow, 2018-2019; currently Lecturer in Biomedical Sciences, The University of York, UK
Kaiwen Chen, Postdoctoral Fellow, 2018-2020; currently Assistant Professor, National University of Singapore (NUS), Singapore
Dora Hancz, Postdoctoral Fellow, 2019-2020; currently Senior Scientist, Novo Nordisk, Sweden
PhD students
Matthias Dick, PhD student, 2013-2017: currently with T3Pharmaceuticals, Basel, Switzerland
Roland Dreier, PhD student, 2013-2017, currently Clinical Trial Scientist Trainee at Idorsia Pharmaceuticals Ltd. Basel, Switzerland
Sebastian Rühl, PhD student, 2014-2018, currently Postdoc St. Jude Children's Research Hospital (Doug Green's Lab)
Rosalie Heilig, PhD student, 2016-2020, currently Postdoc University of Glasgow, UK (Stephen Tait's Lab)
Benjamin Demarco, PhD student, 2018-2021, currently Innovation Postdoctoral Fellow - Novartis, Basel
Kateryna Shkarina, PhD student, 2016-2021, currently Postdoc University of Bonn, Germany (Eicke Latz's Lab)
Other former colleagues
Leonie Anton, Master student (2015-2016) and jointly supervised PhD student with Timm Maier (2016-2017), currently PhD student, Biozentrum, University of Basel, Switzerland (Timm Maier Lab)
Maj Brodmann, Rotation student, 2015, currently PhD student, Biozentrum, University of Basel, Switzerland (Marek Basler Lab)
Anne Kistner, Summer student, currently PhD student, Department of Biomedicine, University of Basel, Switzerland