HELD Lab
Our focus
Cytotoxic CD8 T cells contribute to immune protection against infection with numerous intracellular pathogens but fail to protect against certain pathogens and cancer. We are working on bettering our understanding of the difference between protective and non-protective CD8 T cell responses in order to induce or restore protective immunity.
Our projects
CD8 T cell mediated protection from infection
Immune protection from intracellular pathogens depends on the massive expansion of antigen-specific CD8 T cells and their differentiation into effector cells. In addition, acute resolved infections generate memory CD8 T cells, which persist long-term in the absence of antigen and which rapidly regenerate effector and memory cells upon re-infection.
We found that the transcription factor T cell factor 1 (Tcf1, encoded by the Tcf7 gene) is selectively required for the formation of central memory i.e. memory T cells that can re-expand and regenerate effector and memory cells upon re-infection. (Jeannet et al 2010 PNAS). Ongoing projects address how Tcf1 signaling is controlled during a primary CD8 T cell response. Maintaining Tcf1 signaling may improve the formation of central memory during vaccination or for therapies designed to promote sustained cytotoxic CD8 T cell responses against tumors.
Failure of CD8 T cell mediated protection against infection
Failure to control certain infections leads to the co-existence of pathogen replication and an immune response. Such chronic infections promote the terminal differentiation or “exhaustion” of T cells and were thought to preclude the formation of memory.
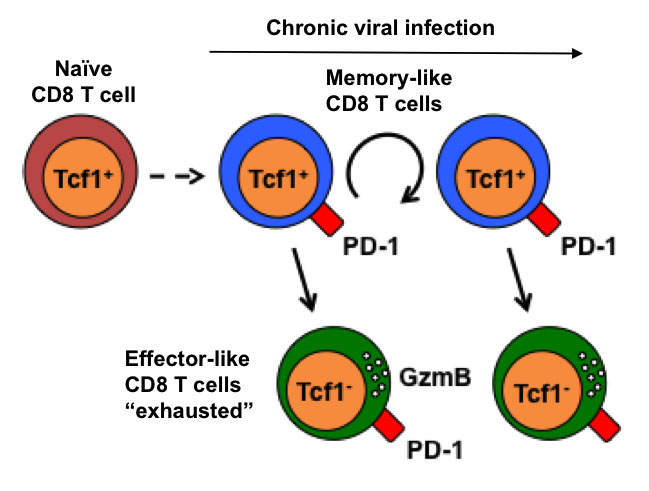
However, we recently discovered a small subpopulation of virus-specific CD8+ T cells that sustained the T cell response during chronic infections. This population shared key characteristics of central memory cells together with hallmarks of an “exhausted” phenotype, including the expression of inhibitory receptors such as PD-1. This memory-like population was crucial for CD8 T cell expansion that occurred in response to inhibitory receptor blockade during chronic infection (Utzschneider et al 2016 Immunity). Ongoing projects address how memory-like CD8 T cells are generated and maintained and whether similar cells are present in tumor immune responses.
We identified a unique population of Tcf1+ CD8 T cells that sustains the immune response during chronic infection. These memory-like CD8 T cells share characteristics of central memory and exhausted cells (PD-1 expression) but not effector cells. They can self-renew and produce effector-like (exhausted) T cells and they expand when the inhibitory receptor PD-1 is blocked.
Normal hematopoiesis and leukemia
Tcf1 can function as a transcriptional repressor or as a nuclear effector of the canonical Wingless/Integration 1 (Wnt) signaling pathway. Since Tcf1 is required for the generation of stem cell like CD8 T cells in acute and chronic infections we also addressed whether the canonical Wnt signaling pathway played a role for hematopoietic stem cells. Unexpectedly we found that that steady-state haematopoiesis occurred normally in the absence of canonical Wnt signaling i.e. in the combined absence of b-catenin and g-catenin (Jeannet et al 2010 Blood). An ongoing project addresses the role of components of the canonical Wnt pathway for the induction and/or the maintenance of acute lymphoblastic leukemia.
NK cell responses to stressed and transformed cells
NK cells represent the innate counterpart of CD8+ T cells and play important roles to control stressed and transformed cells. Germ-line encoded activation and inhibition receptors enable NK cells to kill when the expression of specific self-ligands on other cells deviates from normal. Unexpectedly, we found that the inhibitory Ly49A receptor not only interacted with MHC-I molecules expressed on other cells (in trans), but also with the MHC-I molecules expressed by the NK cell (in cis). Cis association restricted the number of inhibitory Ly49A receptors available to bind MHC-I on target cells and thus lowered the threshold at which NK cell activation exceeded inhibition (Doucey et al., 2004). Distinct conformations defined the structural basis for cis–trans binding by Ly49 receptors (Back et al., 2009). Additional cell surface receptors were subsequently found to mediate cis–trans binding (Held and Mariuzza, 2008).
NK cell education
NK cells expressing inhibitory MHC-I receptors respond efficiently to activation stimuli, while NK cells lacking MHC-I receptors respond poorly. The mechanism for this NK cell “education” is not well understood but it allows efficient NK cell reactions to host cells lacking MHC-I molecules. Since the inhibitory Ly49A receptor bound MHC-I on the NK cell itself (in cis) we tested whether cis interaction played a role for education. To address this issue we designed a Ly49A variant, which can bind MHC-I molecules expressed on other cells but not those expressed in cis. While this Ly49A receptor variant readily inhibited NK cell effector function, it failed to educate NK cells (Chalifour et al. 2009). These data dissociated the classical inhibitory from an educating function of an inhibitory receptor and suggested that cis interaction of Ly49A was necessary for NK cell education.
KEY PUBLICATIONS

-
Siddiqui, I., Schaeuble, L., Chennupati, V., Fuertes Marraco, S. A., Calderon-Copete, S., Pais Ferreira, D., Carmona, S. J., Scarpellino, L., Gfeller, D., Pradervand, S., Luther, S. A. Speiser, D. E. and Held, W. 2019. Intratumoral Tcf1+ PD-1+ CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity. 50, 195–211. PMID: 30635237
-
Luong-Gardiol, N., Siddiqui, I., Pizzitola, I., Jeevan-Raj, B., Charmoy, M., Huang, Y., Irmisch, A., Curtet, S., Angelov, G.A., Danilo, M., Juilland, M., Bornhauser, B., Thome, M., Hantschel, O., Chalandon, Y., Cazzaniga, G., Bourquin, J.-P., Huelsken, J., and Held, W. 2019. y-catenin-dependent signals maintain BCR-ABL1+ B cell acute lymphoblastic leukemia. Cancer Cell. 35, 649-663. PMID: 30991025.
-
Pais Ferreira, D., Gomes Silva, J., Wyss, T., Fuertes Marraco, S. A., Scarpellino, L., Charmoy, M., Maas, R., Siddiqui, I., Tang, L., Joyce, J. A., Delorenzi, M., Luther, S.A., Speiser, D.E. and Held, W. (2020). Central memory CD8+ T cells derive from stem-like Tcf7hi effector cells in the absence of cytotoxic differentiation. Immunity. 53: 985-1000. PMID: 33128876
Meet all the Held Lab Members.