VANNINI Lab
Our focus
We are focussed on identifying the metabolic pathways regulating hematopoietic stem and immune cells function. Our goal is to develop targeted metabolic therapies capable of preserving hematopoietic stem and immune cell functions in the context of aging and chemotherapeutic treatments.
Our projects
Metabolic reprogramming of aged hematopoetic stem and immune cells
Aging is associated with the decline of immune function, especially T-cell mediated activity, which contributes to a reduced tumor immunosurveillance and thus increased tumor insurgences with age. Lymphoid progenitor cells are formed in the bone marrow from hematopoietic stem cells (HSCs) and aging processes profoundly perturb HSCs function. Aging primes HSCs toward myeloid lineages and thus reduces the lymphoid compartment and compromises immune-reconstitution and immunosurveillance.
Over the past years, metabolism has been emerging as major regulator of stem and immune cells function and activation. Recently, it has been shown that metabolic interventions both in animal model and in human can profoundly alter stem and immune cell function. In light of this, we are interested in identifying the metabolic changes happening in HSCs and immune system during aging and developping targeted metabolic therapies in order to re-program stem and immune function in the elderly.
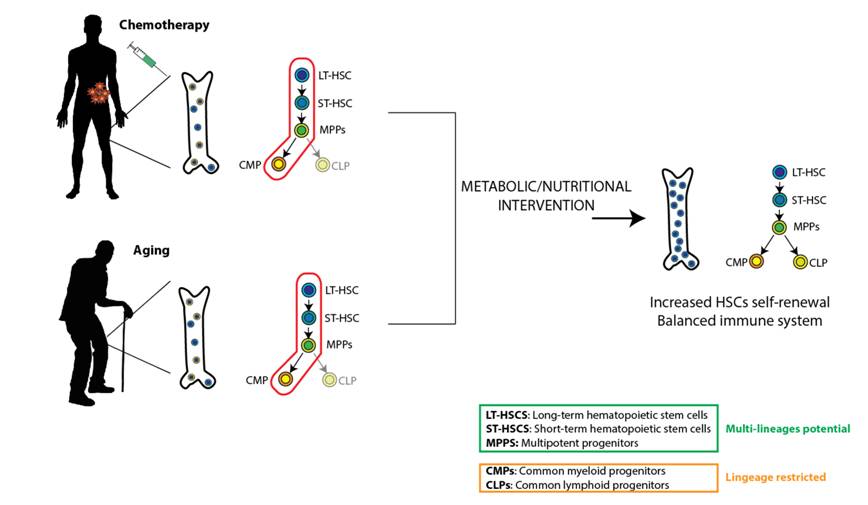
Metabolic prevention of chemotherapy-induced immunosuppression
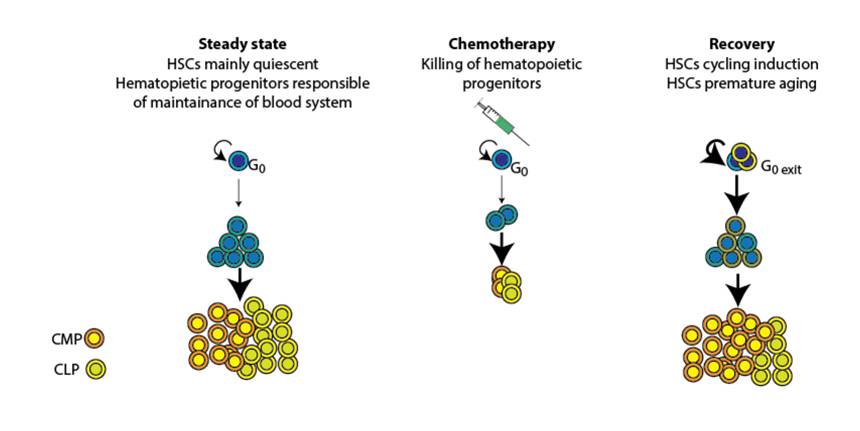
Chemotherapy results in substantial damage to the immune system by affecting the hematopoietic stem and progenitor cells (HSPCs) function, leading to myeloid bias differentiation and subsequent immunosuppression. We are interested in understanding the metabolic changes occuring upon chemotherapy in HSPCs and T cells and developping metabolic/nutritional strategies capable of preserving stem and immune cell functions.
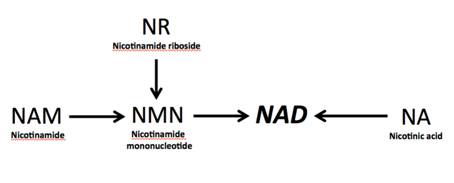
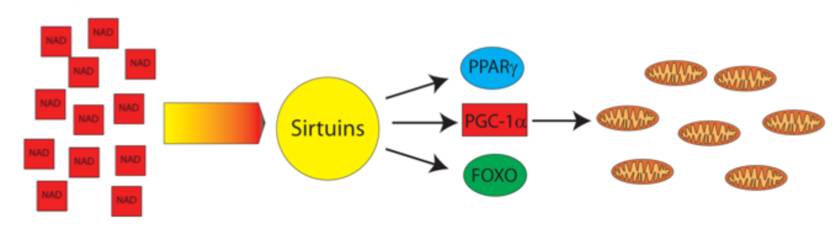
NAD metabolism in immune cells
NAD precursor nicotinamide riboside (NR) has been shown to protect against high fat diet-induced obesity and extend lifespan. Interestingly, in our previous studies we demonstrated that NR is capable to expand HSPCs and lymphoid compartments in vivo (Vannini et al., Under revision) Following up this earlier research, our goal now is to understand the role of NAD metabolism in immune cells.
KEY PUBLICATIONS

- Mukul Girotra, Yi-Hsuan Chiang, Melanie Charmoy, Pierpaolo Ginefra, Helen Carrasco Hope, Charles Bataclan, Yi-Ru Yu, Frederica Schyrr, Fabien Franco, Hartmut Geiger, Stephane Cherix, Ping-Chih Ho, Olaia Naveiras, Johan Auwerx, Werner Held, Nicola Vannini. Induction of mitochondrial recycling reverts age-associated decline of the hematopoietic and immune systems. Nature Aging (2023)
- Konz T, Monnard C, Rincon Restrepo M, Laval J, Sizzano F, Girotra M, Dammone G, Palini A, Coukos G, Rezzi S, Godin JP, Vannini N. Multi-elemental analysis of low-volume samples reveals cancer-specific profile in serum and sorted immune cells. Analytic Chemistry (2020)
- Yu YR, Imrichova H, Wang H, Chao T, Xiao Z, Gao M, Rincon-Restrepo M, Franco F, Genolet R, Cheng WC, Jandus C, Coukos G, Jiang YF, Locasale JW, Zippelius A, Liu PS, Tang L, Bock C, Vannini N and Ho PC. Disturbed mitochondrial dynamics in CD8+ TILs reinforce T cell exhaustion. Nature Immunology (2020)
- Vannini N, Campos V, Girotra M, Trachsel V, Rojas-Sutterlin S, Tratwal J, Ragusa S, Stefanidis E, Ryu D, Rainer PY, Nikitin G, Giger S, Li TY, Semilietof A, Oggier A, Yersin Y, Tauzin L, Pirinen E, Cheng WC, Ratajczak J, Canto C, Ehrbar M, Sizzano F, Petrova TV, Vanhecke D, Zhang L, Romero P, Nahimana A, Cherix S, Duchosal MA, Ho PC, Deplancke B, Coukos G, Auwerx J, Lutolf MP, Naveiras O*. The NAD-Booster Nicotinamide Riboside Potently Stimulates Hematopoiesis through Increased Mitochondrial Clearance. Cell Stem Cell (2019)
Meet all the Vannini Lab Members.
| Funding |
|
|
Affiliations |
|







